We always think of our customers with an open mind
Press Release
NTL Healthcare Receives Approval from the Ministry of Food and Drug Safety for Its Cervical Cancer Artificial Intelligence Diag
Author
엔티엘헬스케어
Date
2023-05-31 11:49
Views
235
NTL Healthcare Receives Approval from the Ministry of Food and Drug Safety for Its Cervical Cancer Artificial Intelligence Diagnostic Device
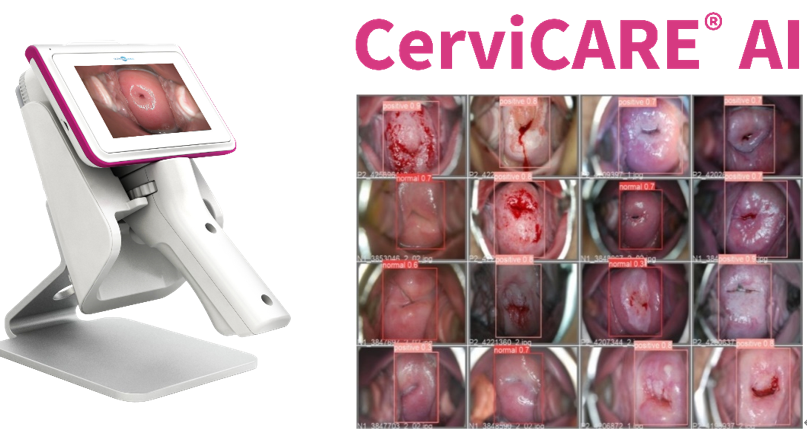
NTL Healthcare, Korean obstetrics and gynecology specialized company, which has led the global early diagnosis market for cervical cancer, announced on the 12th that it has obtained a Class 3 approval for its obstetrics and gynecology medical treatment software, "CerviCARE® AI," from the Ministry of Food and Drug Safety. With this achievement, NTL aims to solidify its position as a leader in the field of cervical cancer screening.
CerviCARE® AI is a cervical cancer diagnosis assisting software developed by NTL Healthcare using big data from cervical images accumulated over the past 27 years, in collaboration with the NTL Medical Institute. It generates results based on an automatic reading algorithm for the captured cervical images and provides real-time information to the medical professionals, enabling efficient and accurate cervical cancer diagnosis.
NTL registered a patent for an "Artificial Intelligence (AI)-based cervical cancer screening service system" in December of last year. In addition, NTL has also completed the patent registration for a "cervical diagnostic camera device with reduced light reflection technology" for accurate cervical cancer diagnosis.
The device is equipped with high brightness lighting for sharp image quality, which reduces visual interference caused by the light reflex of cervical mucus. In addition, the device includes a design feature that considers user convenience, with a UI and UX optimized for capturing images of the cervix, vulva, and vagina.
In order to expand its overseas business, NTL is focusing on the South American market, centered on Brazil, as well as the African, Chinese, Southeast Asian, and Middle Eastern markets. For the existing business, the cervical magnification imaging and reading system (TeleCervico), NTL is waiting for approval from the Brazilian Health Regulatory Agency (ANVISA) for registration. The system is planned to be supplied nationwide in Brazil, starting with the medical school of the University of Sao Paulo.
Tae-hee Kim, CEO of NTL Healthcare, said, "We plan to proceed with the application for new medical technology assessment of CerviCARE® AI and the safety and efficacy assessment process of new medical technology of the National Evidence-based Healthcare Collaborating Agency (NECA)." She added, "Our service is highly recognized in overseas markets where remote medical services are becoming more popular, due to its easy accessibility, quick and accurate diagnosis prediction," and said, "We will contribute to improving the quality of life for women around the world with our differentiated service."

NTL Healthcare, Korean obstetrics and gynecology specialized company, which has led the global early diagnosis market for cervical cancer, announced on the 12th that it has obtained a Class 3 approval for its obstetrics and gynecology medical treatment software, "CerviCARE® AI," from the Ministry of Food and Drug Safety. With this achievement, NTL aims to solidify its position as a leader in the field of cervical cancer screening.
CerviCARE® AI is a cervical cancer diagnosis assisting software developed by NTL Healthcare using big data from cervical images accumulated over the past 27 years, in collaboration with the NTL Medical Institute. It generates results based on an automatic reading algorithm for the captured cervical images and provides real-time information to the medical professionals, enabling efficient and accurate cervical cancer diagnosis.
NTL registered a patent for an "Artificial Intelligence (AI)-based cervical cancer screening service system" in December of last year. In addition, NTL has also completed the patent registration for a "cervical diagnostic camera device with reduced light reflection technology" for accurate cervical cancer diagnosis.
The device is equipped with high brightness lighting for sharp image quality, which reduces visual interference caused by the light reflex of cervical mucus. In addition, the device includes a design feature that considers user convenience, with a UI and UX optimized for capturing images of the cervix, vulva, and vagina.
In order to expand its overseas business, NTL is focusing on the South American market, centered on Brazil, as well as the African, Chinese, Southeast Asian, and Middle Eastern markets. For the existing business, the cervical magnification imaging and reading system (TeleCervico), NTL is waiting for approval from the Brazilian Health Regulatory Agency (ANVISA) for registration. The system is planned to be supplied nationwide in Brazil, starting with the medical school of the University of Sao Paulo.
Tae-hee Kim, CEO of NTL Healthcare, said, "We plan to proceed with the application for new medical technology assessment of CerviCARE® AI and the safety and efficacy assessment process of new medical technology of the National Evidence-based Healthcare Collaborating Agency (NECA)." She added, "Our service is highly recognized in overseas markets where remote medical services are becoming more popular, due to its easy accessibility, quick and accurate diagnosis prediction," and said, "We will contribute to improving the quality of life for women around the world with our differentiated service."